Discover CIMERLI®
An FDA-approved treatment for retinal conditions1
Understanding your eye condition
If you've been diagnosed with
- Wet age-related macular degeneration (wAMD)
- Diabetic retinopathy and/or diabetic macular edema (DR, DME)
- Myopic choroidal neovascularization (mCNV)
- Macular edema following retinal vein occlusion (RVO)
you may be experiencing issues with your vision.
CIMERLI® is a prescription medicine for the treatment of patients with any of the conditions listed above.
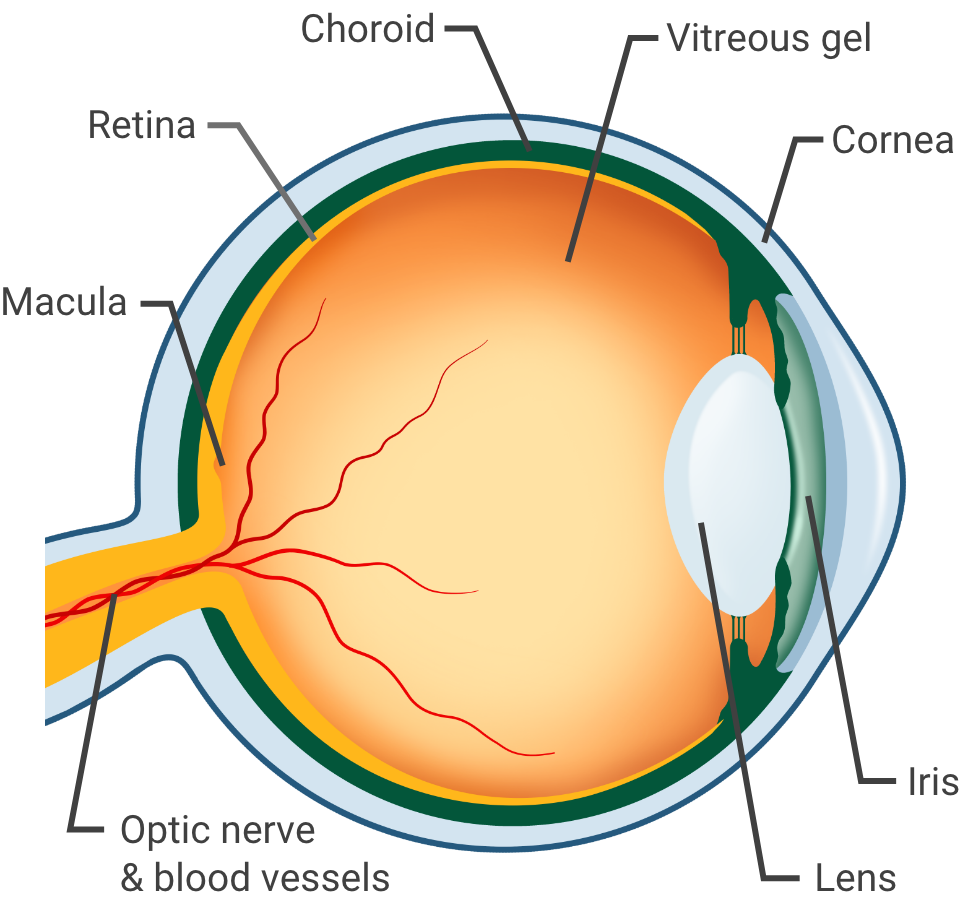
Healthy eye
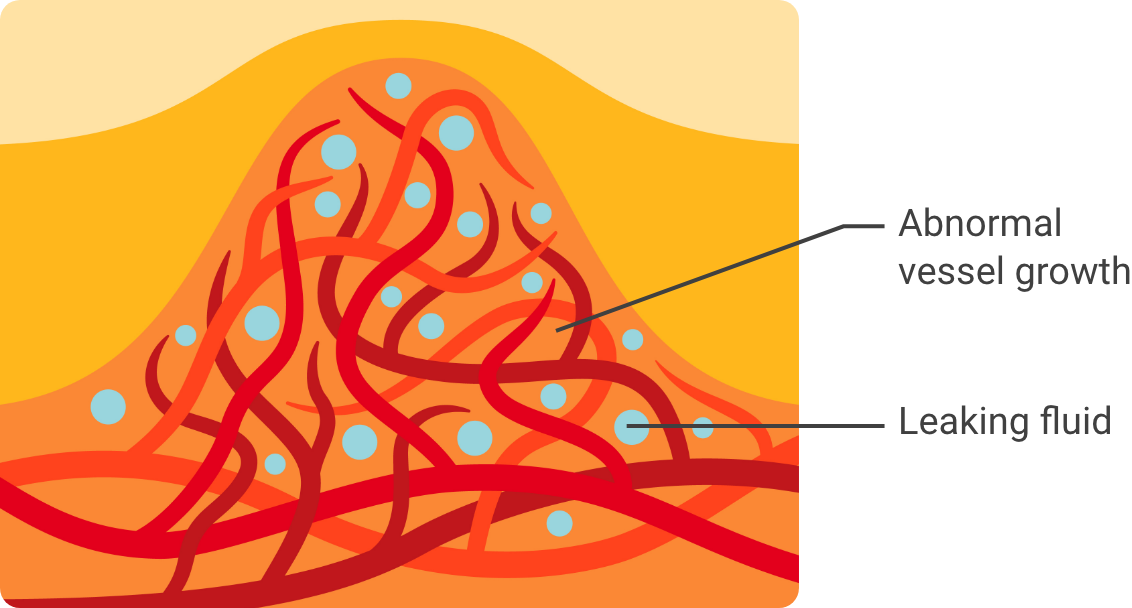
In these conditions, problems with eyesight can occur when:
Abnormal blood vessels grow in a part of the eye known as the retina

Healthy blood vessels

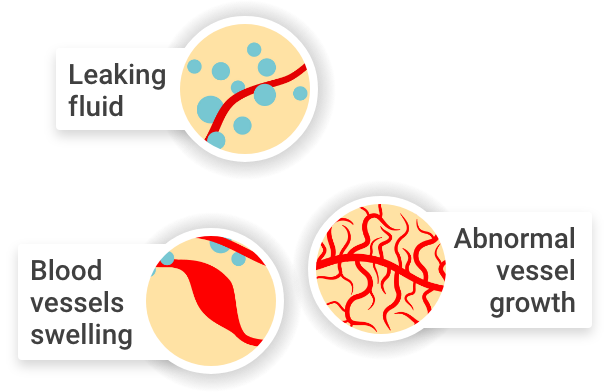
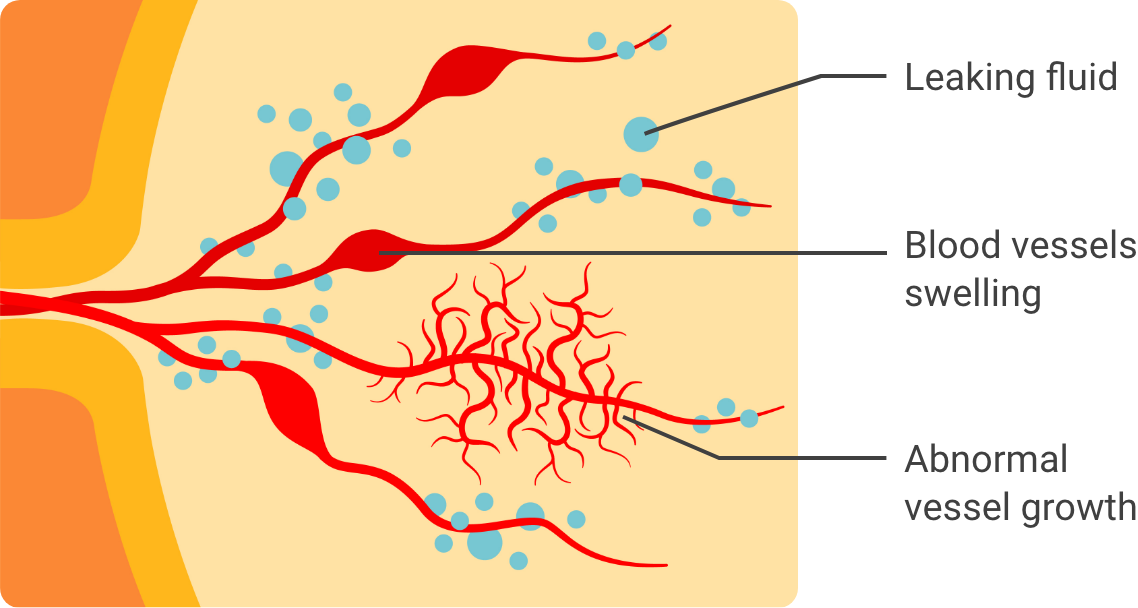
Abnormal blood vessels
View
Labels
Or, when these blood vessels leak fluid into an area of the retina called the macula

Healthy macula

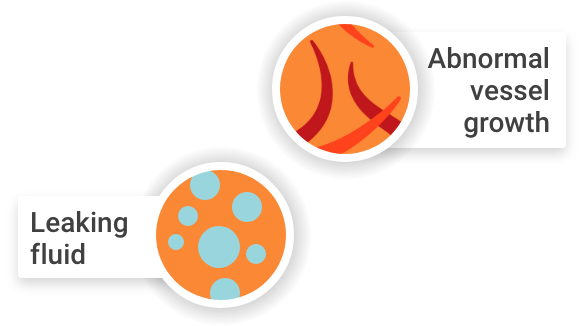
Swollen macula
View
Labels
What is CIMERLI®?
CIMERLI® is an FDA-approved biosimilar that is interchangeable with Lucentis® (ranibizumab injection)2
CIMERLI® is a biosimilar—a type of biologic medicine that is highly similar to the FDA-approved biologic Lucentis®.3
CIMERLI® can be used to treat the same eye conditions as Lucentis®, and as an interchangeable biosimilar, it is expected to have the same effectiveness and safety, which makes it possible to be directly substituted for Lucentis®.1,3
With no clinically meaningful differences between the two medicines3, your doctor can choose to start or transition you to CIMERLI® without impacting your treatment.

Why are biosimilars important?4,5
Why are biosimilars important?4,5
Biosimilars were established to
- Help reduce out-of-pocket & medication costs for patients
- Make important treatments accessible to patients who need them
- Potentially help reduce healthcare spending
all while maintaining the same quality, safety, and effectiveness of treatment.
What you can expect from CIMERLI®1,3
Your doctor has chosen to start or transition you to CIMERLI®. Here are some things you may find helpful to know:

The same treatment approach
Both treatments are given as injections to the eye and are dosed the same way. Treatment is typically recommended monthly, but your doctor will determine a dosing schedule that is appropriate for you.

The same way of working
Both treatments work the same way in the eye and are expected to produce the same clinical results.

The same effectiveness & safety
CIMERLI® can be substituted for Lucentis without compromising treatment impact or safety. CIMERLI® was carefully reviewed and thoroughly evaluated by a rigorous FDA-approval process, and it is required to meet high FDA standards for consistent manufacturing to ensure medication quality and safety.

Financial support is available
Sandoz One Source® for CIMERLI® offers a range of financial assistance programs for patients who are prescribed CIMERLI®.
Co-Pay Savings Program
The Co-Pay Savings Program may cover out-of-pocket expenses associated with CIMERLI® and the injection procedure for eligible patients with commercial insurance*:
per dose of CIMERLI® including injection
with a maximum annual benefit of $16,000 per calendar year*
per dose of CIMERLI® including injection with a maximum annual benefit of $16,000 for drug costs per calendar year*
Talk to your doctor’s office to make sure you’re enrolled in Sandoz One Source so you can access all the program benefits
*Patient Drug and Injection Co-Pay Eligibility Criteria:
- Be prescribed CIMERLI® for a medically appropriate purpose consistent with its FDA-approved labeling within 180 days of program enrollment
- Have commercial (private or non-governmental) health insurance that covers the medication costs of CIMERLI®
- Not covered by any federal, state, or government-funded healthcare program, such as Medicare, Medicare Advantage, Medicare Part D, Veterans Affairs, Department of Defense, or TRICARE
- Not seek reimbursement from any third party, including payers, charitable foundations, or flexible spending accounts (FSAs ) or healthcare savings accounts (HSAs) for all or any part of the benefit received by Sandoz through this program
- Other restrictions apply, see Terms & Conditions (opens in a new tab)
- It is not valid for cash-paying patients or where prohibited by law
- Co-Pay Savings Program subject to change or discontinuation without notice. This is not health insurance
Sandoz Patient Assistance (SPA)†
CIMERLI® may be available to you at no cost if you are uninsured or functionally underinsured.
Independent Foundation Support
Sandoz One Source may be able to help you find financial support through charitable foundations, or you can choose to contact these independent assistance foundations directly.
Call 1-844-4SANDOZ
(1-844-472-6369) Monday–Friday 8 AM to 8 PM ET for any questions related to financial support
†To be eligible for SPA assistance, you must:
- Reside in the United States or a U.S. Territory
- Have limited or no prescription insurance coverage
- Meet income guidelines adjusted for household size, for the medication for which the patient is seeking assistance
- Have a valid prescription for the Sandoz medication
- Be treated by a licensed U.S. healthcare provider
- Complete and sign consent form and, when applicable, provide income documentation
Learn more about CIMERLI®
The patient brochure includes more information about CIMERLI®, biosimilars, and the financial support that may be available to you.
Download the CIMERLI® Patient Brochure (opens in a new tab) Download the CIMERLI® Patient Brochure (opens in a new tab)
Download the CIMERLI® Patient Brochure (opens in a new tab)References:
- CIMERLI™ (ranibizumab-eqrn) prescribing information. Princeton, NJ: Sandoz, Inc.; 2024.
- Purple Book Database of Licensed Biological Products. U.S. Food and Drug Administration. https://purplebooksearch.fda.gov/faqs (opens in a new tab). Updated 2024. Accessed on June 4, 2024.
- Biosimilar and Interchangeable Products. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices (opens in a new tab). Published October 23, 2017. Accessed on May 19, 2022.
- The U.S. Generic & Biosimilar Medicines Savings Report. Accessiblemeds.org. https://accessiblemeds.org/sites/default/files/2021-10/AAM-2021-US-Generic-Biosimilar-Medicines-Savings-Report-web.pdf (opens in a new tab). Published 2021. Accessed on February 22, 2022.
- Makurvet F. Biologics vs. small molecules: Drug costs and patient access. Med Drug Discov. 2021;9:100075.
doi:10.1016/j.medidd.2020.100075